Recently, National Medical Products Administration (NMPA) approved the registration application of an innovative product “implantable left ventricular assist system” produced by CH Biomedical, Inc. It is an initiative medical device in China. Its core technology -- full magnetic suspension blood pump technology -- has obtained many patents in China and the United States. Compared with international similar products, this product has equivalent key performance parameters, a smaller blood pump, and more superior implant invasiveness. This product can satisfy the clinical needs of surgical instruments for heart failure in China, and has important social benefits.
▲ The implantable left ventricular assist system was officially approved by NMPA.
(Picture from NMPA)
It is the first domestic artificial heart with complete independent intellectual property rights approved by the NMPA, and also the first full magnetic suspension artificial heart approved. It marks the successful commercialization of the ventricular assist devices (VAD) developed by a global new generation technical route (full magnetic suspension) in China, and will open a new era of heart failure treatment in China.
Tigermed-Jyton Contributes to Successful Launch of an Artificial Heart with Professional Clinical Trial Services
Tigermed-Jyton provided operation management of clinical trials for this product. Artificial hearts pose higher professional requirements for CRO teams compared with other medical devices. The clinical teams of artificial hearts should not only have excellent monitoring quality and rich monitoring experience, but also master professional knowledge of cardiac surgery, and continuously study and explore according to the progress of clinical trials. The clinical trial of this product was amid the most severe period of the COVID-19 pandemic, and the data to be processed was tremendous. Nevertheless, the clinical trial was successfully completed with the intense cooperation of investigators and the unremitting efforts of the clinical team of Tigermed-Jyton. Tigermed-Jyton contributed to the rapid launch of the product.
A New Generation of Full Magnetic Suspension Artificial Heart Reaching the International Leading Level
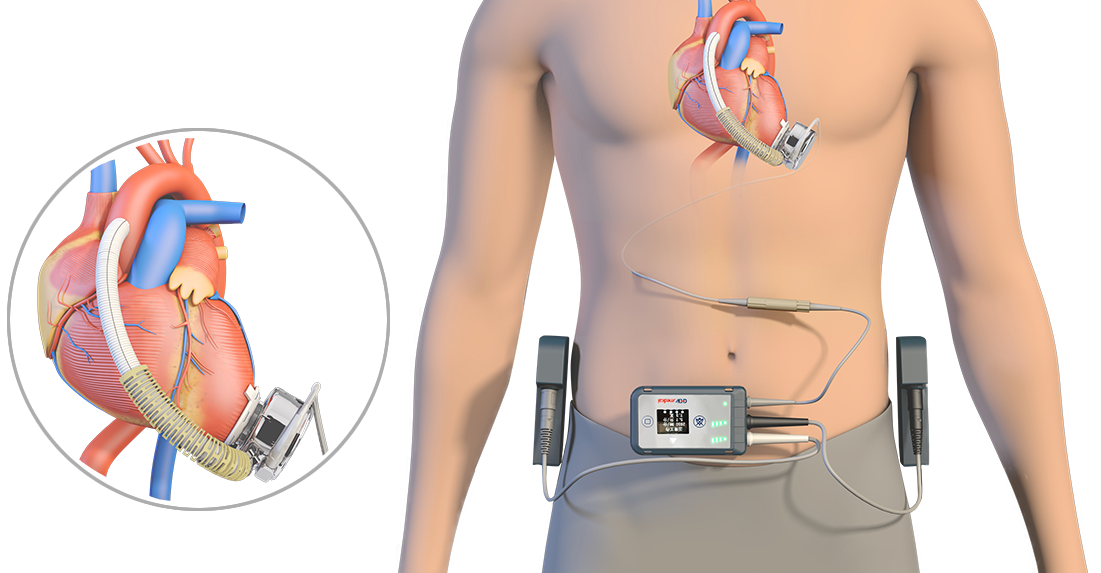
CH-VAD® implantable left ventricular assist system is a new generation of full magnetic suspension artificial heart, which consists of the implantable part and the extracorporeal part. The core component -- blood pump is a special and precise centrifugal pump which can be held in the palm of your hand. Its impeller is supported by a magnetic bearing and rotates driven by the brushless direct current motor. By rotating the blade rotor, negative pressure is formed in the center of the blood pump rotor to pump the left ventricular blood of the patient to the ascending aorta, and flow is generated in the circulatory system, so that the blood pump and the natural heart can work collaboratively. Functions such as power management, blood pump status monitoring and adjustment can be realized with the extracorporeal controller, and a user interface is also provided. The entire system, incorporating both hardware and software, shall meet extremely high requirements of reliability, safety and electromagnetic compatibility. Relevant tests demonstrate that the product has reached the international leading level in key performance parameters such as blood compatibility, implant invasiveness and reliability.