On April 25, 2023, the Center for Medical Device Evaluation, NMPA, Hainan Medical Products Administration and Lecheng Administration jointly formulated and issued the Implementation Measures for the Pre-communication of Clinical Real-World Data Application of Medical Devices in Hainan Boao Lecheng International Medical Tourism Pilot Zone (Trial) (hereinafter referred to as the Implementation Measures), which has been implemented it recently. The issuance of the Implementation Measures is a great boost for the real-world study of Boao Lecheng licensed drugs and medical devices for product registration.
The issuance of the Implementation Measures is a continuous innovation and deepening on the basis of “real-world study pilot”, which promotes the normalization and institutionalization of real-world study of Lecheng licensed drugs and medical devices for product registration and launching. It expands the service scope of pre-communication, determines that the pre-communication is conducted by Hainan Medical Products Administration, improves the efficiency of pre-communication application, clarifies the requirements for materials of pre-communication application, and provides the policy convenience for manufacturers through the real-world study for product registration.
I. Expanding the service scale of pre-communication
The Implementation Measures specify that “These Measures are applicable to the products that belong to the imported medical devices urgently needed in clinical practice according to the Provisions on Administration of Imported Drugs and Medical Devices Urgently Needed in Clinical Practice in Boao Lecheng International Medical Tourism Pilot Zone, Hainan Free Trade Port, which will be carried out with the real-world study of medical devices for product registration in Lecheng International Medical Tourism Pilot Zone and confirmed by Hainan Medical Products Administration to be included in pre-communication service.” That is to say, all licensed drugs and medical devices that will be carried out with the real-world study in Boao Lecheng can be applied for pre-communication service and can be included in the scope of pre-communication service after approval of formal examination by Hainan Medical Products Administration. Compared with the previous “real-world study pilot" stage with only 10 places per year, the service scale has been greatly expanded.
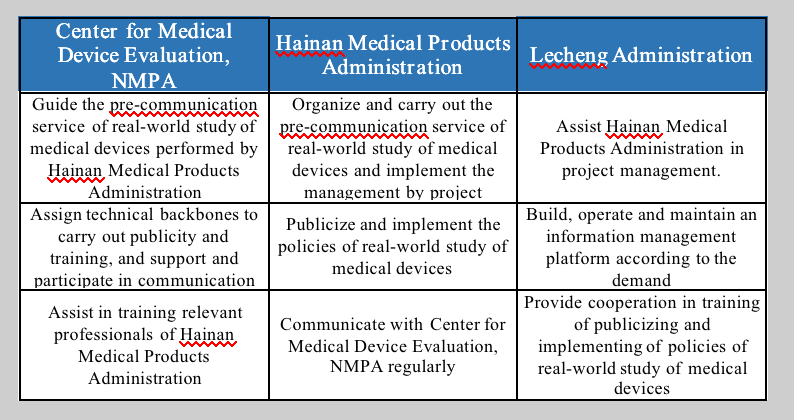
II. Clarifying the responsibilities of all parties and improving the communication efficiency
The Center for Medical Device Evaluation, NMPA, Hainan Medical Products Administration and Lecheng Administration are linked, with clear responsibilities. Hainan Medical Products Administration will assume the responsibility of pre-communication service. It will strictly review the materials to ensure that the materials meet the review requirements. When encountering more complicated technical problems, Hainan Medical Products Administration will also seek technical guidance from the National Medical Products Administration to help the products obtain the registration acceptance of Center for Medical Device Evaluation, NMPA from the bottom up.
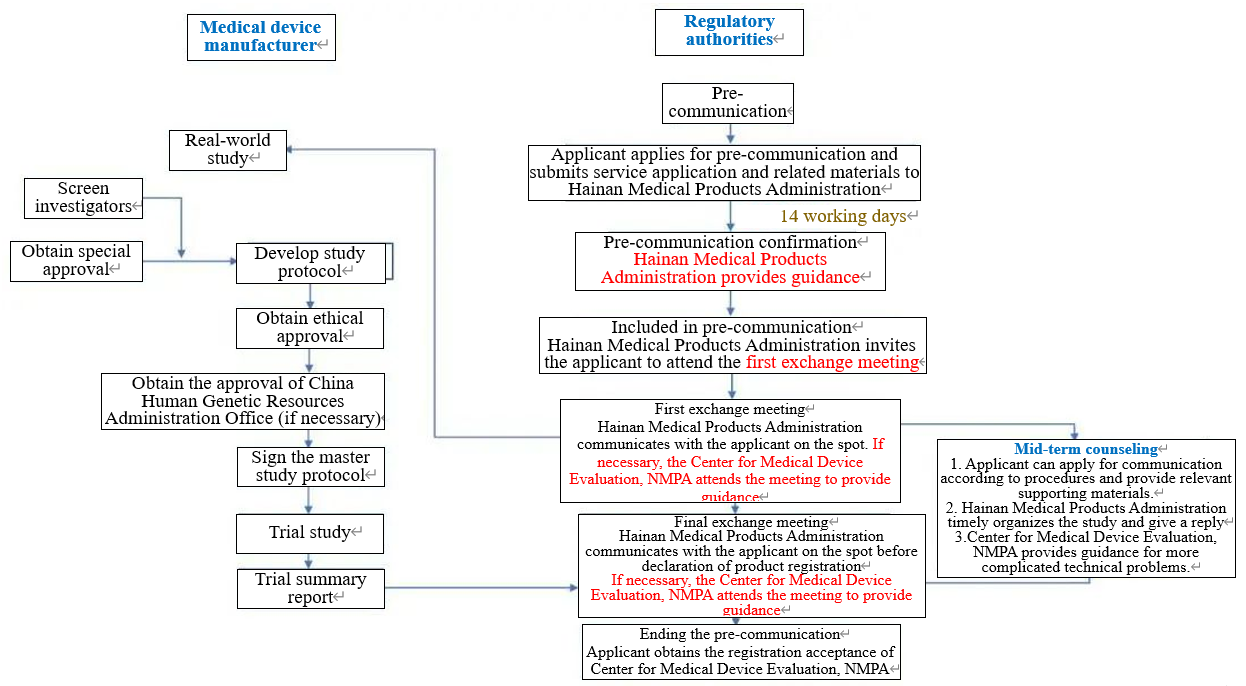
3. Optimizing the workflow and shortening the confirmation time of pre-communication service
After the manufacturer submits the relevant materials as required, Hainan Medical Products Administration will give the final confirmation within 14 working days. For the products confirmed to be included, the pre-communication process will be completed according to the first exchange meeting, mid-term counseling and the final exchange meeting.
Hainan Medical Products Administration has issued a guideline on May 19 for the application materials submitted for pre-communication. Applicants shall submit the relevant materials through Hainan real-world data platform and the materials submitted shall be accurate and complete. In the basic product materials, focus will be on the consistency, compliance and accuracy of the contents involved in the materials. Product name, model and specification, factory name and address, scope of application, etc. shall be consistent; product naming, basis of product launching, authorization documents, etc. shall meet the requirements specified in regulations, and; product review, principle and composition, conclusion of clinical study, conclusion of race difference, adverse reactions, etc. shall be consistent with the actual situation, avoiding false declaration and concealed declaration. The real-world study materials mainly elaborate on the problems solved by real-world study from the perspectives of scientificity, standardization, operability and ethical compliance. The accuracy and completeness of materials is an important condition for Hainan Medical Products Administration to improve the review efficiency.
The implementation of the real-world study for Boao Lecheng licensed drugs and medical devices for product registration and launching has experienced several important historical moments. In September 2019, the National Development and Reform Commission, National Health Commission of People's Republic of China, National Administration of Traditional Chinese Medicine and National Medical Products Administration jointly issued the Notice on Issuing the Implementation Protocol for Supporting the Construction of Lecheng International Tourism Pilot Zone, encouraging the real-world study carried out in Lecheng Pilot Zone, and specifying that the compliant clinical use data of a small quantity of imported drugs and medical devices urgently needed in clinical practice in Lecheng Pilot Zone can be used for registration application of imported drugs and medical devices. This Implementation Protocol provides a policy guarantee for Lecheng licensed drugs and medical devices to carry out real-world study for product registration application. In October 2019, the National Medical Products Administration and Hainan Medical Products Administration hosted a seminar on clinical real-world medical device pilot products and identified the first batch of 10 varieties, truly putting the real-world data application into operation. In March, 2020, the first licensed medical device was approved for market launch through the patten of overseas clinical trial data and real-world study pilot data, making us see that it is possible to carry out real-world study of Boao Lecheng licensed medical devices for product registration.
Up to now, 9 licensed medical devices have been registered and launched, greatly boosting the confidence of international medical manufacturers of drugs and medical devices to continue to increase product launch and carry out real-world study in Lecheng.
Attachment:
https://amr.hainan.gov.cn/himpa/ywdt/gzdt/202305/t20230519_3418865.html
Guidelines for Submission of Pre-Communication Materials for Clinical Real-World Data Application of Medical Devices in Hainan Boao Lecheng International Medical Tourism Pilot Zone (Trial)