Tigermed-Jyton, a wholly-owned subsidiary of Tigermed Group (Stock code: 300347.SZ/3347.HK), was established in 2000, and is a leading CRO service provider in China for regulatory affairs and clinical research of medical devices (including in vitro diagnostic products), with service covering the whole life cycle of all fields of medical devices. In the past 20 years, we have established long-term cooperative relations with more than 1,700 medical device R&D and manufacture enterprises from more than 30 countries. With our professional service capability, we can reduce registration risk, shorten project timeline, save R&D funds and accelerate the process of product marketization.
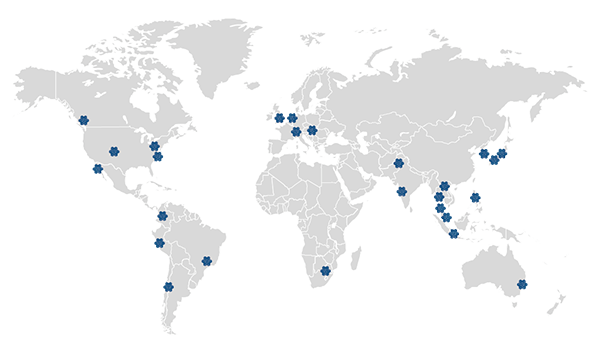
Supported by Tigermed Group, we have more than 180+ offices in major cities on five continents worldwide,covering major medical device markets such as the United States, Europe, and Asia Pacific.

National High-tech Enterprise

ISO9001

CNAS/CMA