Clinical Operation
Overview
Tigermed-Jyton has always been devoted to providing integrated technology and support for clinical trials intended for the registration of medical devices and IVD products. We will support your registration or pre-marketing study from protocol design, center screening, recordation & approval, center startup, monitoring management, data collation, statistical analysis to summary and the end.
To standardize supervision and administration for medical device clinical trials, the National Medical Products Administration (NMPA) released the Good Clinical Practice (No. 25 NMPA Order) on March 23, 2016 and Inspection Items and Judgment Principles for Clinical Trials of Medical Devices on November 28, 2018. With the implementation of various policies, more professional and normative clinical operation teams are required for clinical trials of medical devices.
Basic principles of clinical trials: Scientific, authentic and compliant
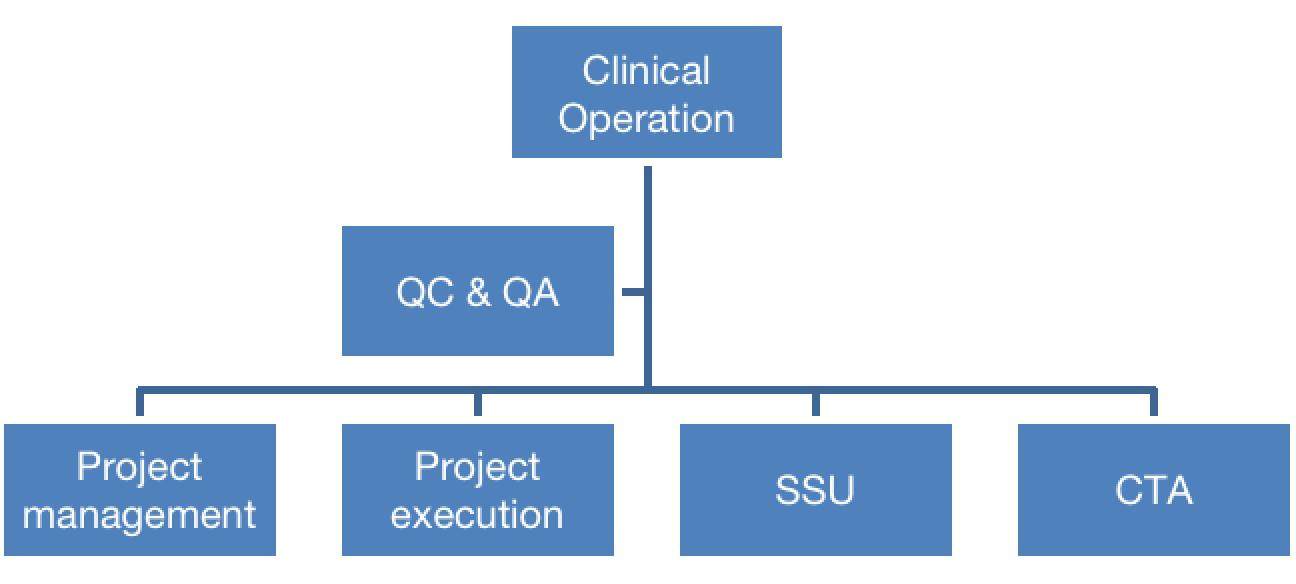
1. Service Contents
SSU: Screening of Clinical Investigation Sites
Project approval of Clinical Investigation Sites
Obtaining ethical approval documents for clinical trials
Project execution: Center startup
Routine monitoring for clinical trials
Investigational product management for clinical trials
Coordination & cooperation of CRC
Project management: Project management for clinical trials
Risk management for clinical trials
Financial management for clinical trials
Organization of clinical trial meetings
Project training
Clinical approval
Recordation with the Genetics Office
CTA: Document management for clinical trials
2. Clinical Operation Guarantee:
Scientific assistance: The project team is guided by senior registration managers & medical managers & the data management & statistics team during the process.
Efficient communication: The status report of the project team and the assistance team is updated every weak, to ensure that all participating departments timely know the project progress, feedbacks and suggestions;
Perfect system: SOP in line with international company standards, regular QC & QA inspection, clear rewards and punishments for document management;
Thoughtful plan: Progress plan and deviation analysis, risk control and prevention, resource allocation, guarantee for scheduled project implementation;
3. Organization Structure of Clinical
Operation: