Quality Control (QC)
Overview
Tigermed-Jyton exercises quality control over clinical trials for medical devices and IVD products, to ensure that the company’s clinical trial projects conform to relevant laws, regulations and SOP of the company.
1. Service contents:
1) Project QC, including QC in clinical trial projects for medical devices and IVD products;
2) Internal and external quality control training
3) Professional GCP training for the sponsor;
4) Professional GCP counseling for research centers;
5) Training for sharing clinical trial and QC experience in an industry association, etc.;
2. Assist Clinical Investigation Sites in filing recordation applications.
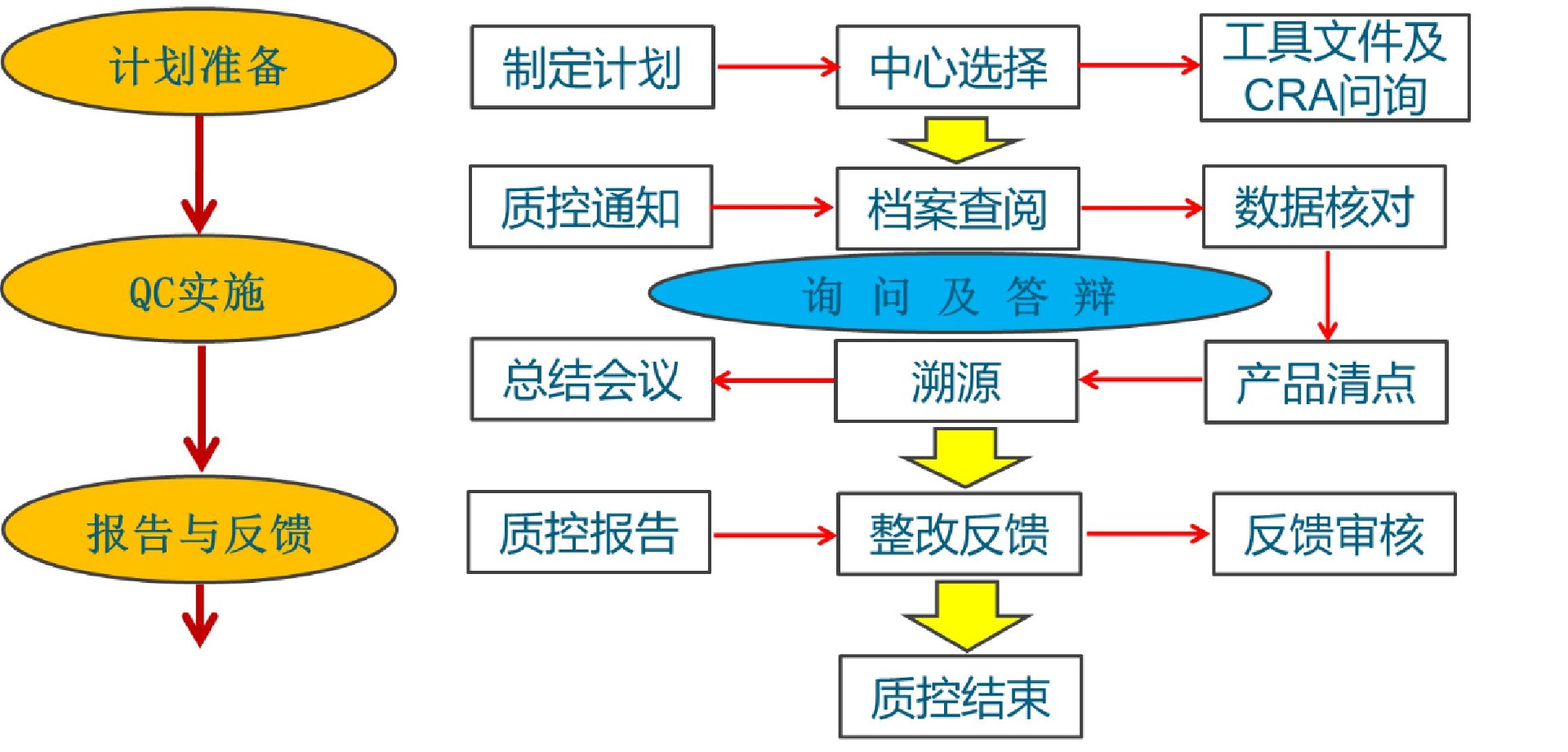
QC process:
No. | Project name | Research center | Review unit | Review date | Review result |
1 | Locking plate system | Shanghai Ninth People’s Hospital | Shanghai Food and Drug Administration | October 2017 | Pass |
2 | Cardiac valve prosthesis made of bovine pericardium | Zhongshan Hospital Affiliated to Fudan University | Shanghai Food and Drug Administration | November 2017 | Pass |
3 | The second-generation dynamic ECG recorder | Xinhua Hospital Affiliated to Shanghai Jiaotong University, Shanghai Municipal Hospital of Traditional Chinese Medicine, Yangpu Hospital | Shanghai Food and Drug Administration | May 2018 | Pass |
4 | Upper limb rehabilitation system | The First Affiliated Hospital of Bengbu Medical College, Liuzhou Municipal Hospital, Nanhua Hospital | Shanghai Food and Drug Administration | November 2018 | Pass |
5 | Aquaporin antibody (AQP4-Ab) detection kit (ELISA) | Beijing Tongren Hospital, Tianjin General Hospital | China Food and Drug Administration | December 2018 | Pass |
6 | Little Shell project | Nanfang Hospital Affiliated to the Southern Medical University, Panyu Central Hospital, Guangzhou Municipal Maternity and Child Care Hospital | Guangzhou Food and Drug Administration | February 2019 | Pass |
7 | COPD | Zhongshan Hospital Affiliated to Fudan University, Shanghai Pulmonary Hospital | Shanghai Food and Drug Administration | May 2019 | Pass |
8 | Type A botulinum toxin for injection | Renmin Hospital of Wuhan University, First Affiliated Hospital of Kunming Medical University | National Medical Products Administration | June 2019 | Pass |
9 | PMTCT assistant software for hepatitis B | Nanfang Hospital Affiliated to the Southern Medical University, The Second Hospital of Nanjing, Guangzhou Women and Children’s Medical Center, Guangzhou Panyu Central Hospital | Zhejiang Medical Products Administration | July 2019 | Pass |