Overview
Clinical Evaluation of Medical Devices refers to that the registration applicant or the filing applicant validates whether the product meets the operating requirements or the scope of application through clinical literature, clinical trial data and clinical trial, etc.
In accordance with the Regulations for the Supervision and Administration of Medical Devices (No. 650 Decree of the State Council), clinical evaluation materials should be provided for medical device registration. Class I medical devices are subject to product recordation and exempted from the clinical trial, but clinical evaluation materials shall be submitted. Clinical trials are required for the registration of Class II and III medical devices (except for those listed in the Catalog of Class II Medical Devices Exempted from Clinical Trials and the Catalog of Class III Medical Devices Exempted from Clinical Trials). Clinical evaluation materials shall be provided for the registration of medical devices exempted from clinical trials.
To implement the Opinions on Deepening Review and Approval System Reform and Encouraging Drug and Medical Device Innovation (T.Zi [2017] No.42) issued by the General Office of the CPC Central Committee and the General Office of the State Council, reinforce the management of medical device registration, further improve the quality of registration and review and encourage medical device R&D innovation, China Food and Drug Administration (CFDA) issued the Technical Guidelines for Accepting Data of Overseas Clinical Trials on Medical Devices on January 11, 2018. As specified in this guideline, overseas clinical trial data can be accepted as the clinical evaluation material when the applicant applies for medical device registration in China.
This guideline stated the ethical, legal and scientific principles of accepting overseas clinical trial data, and defined the material requirements and technical requirements for overseas clinical trial data. This guideline explained factors considered and technical requirements for accepting overseas clinical trial materials from the difference of technical review requirements, subjects and clinical trial conditions, etc., and provided examples for different factors that have a significant influence on clinical data. In accordance with this regulation, the clinical evaluation team has completed the clinical evaluation report with overseas clinical data for many transnational medical device enterprises, to support the registration of their products in China, avoid or reduce repeated clinical trials and accelerate the marketing progress in China.
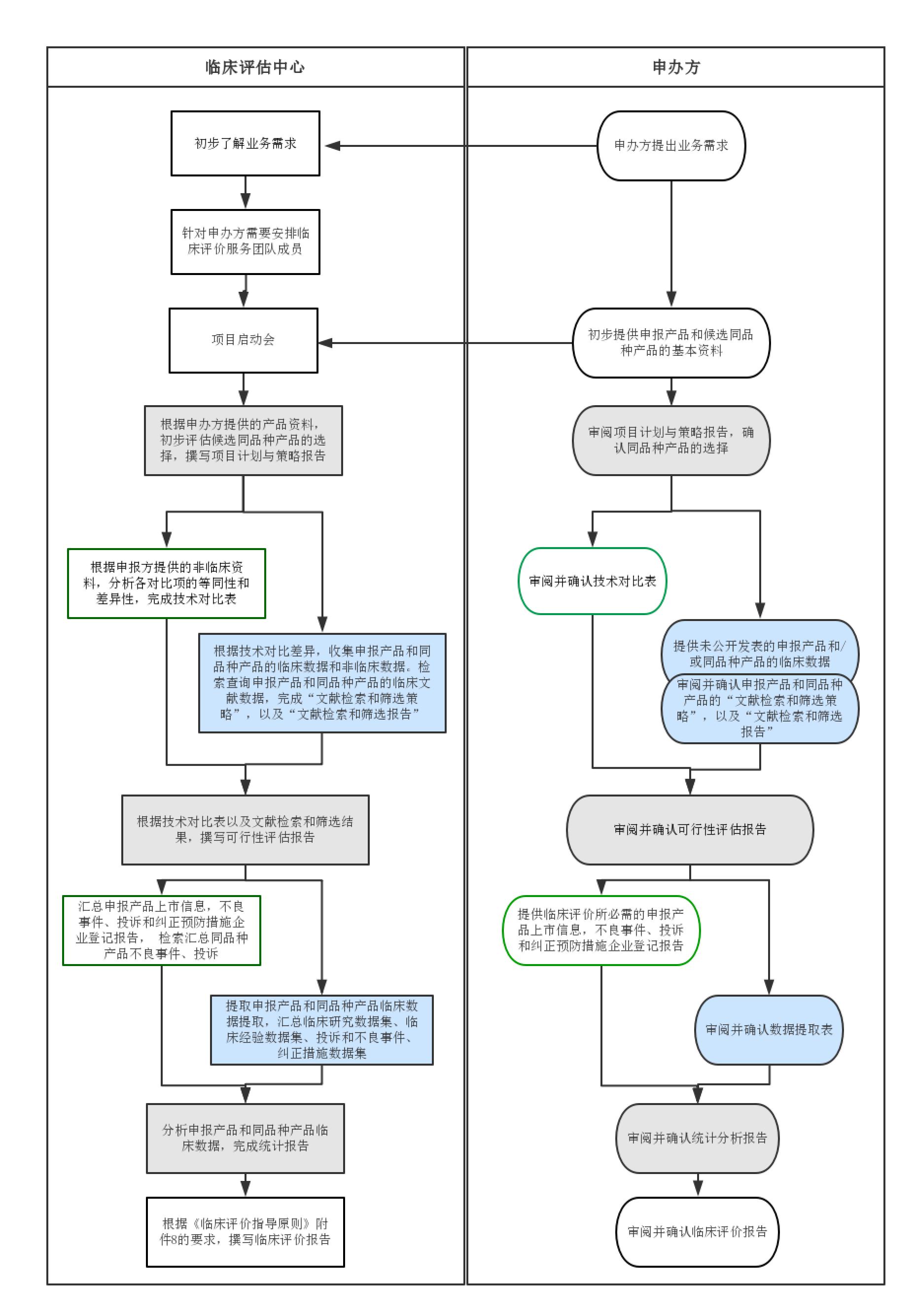
1. Service Team
In May 2015, CFDA formally issued Technical Guidelines for Clinical Evaluation of Medical Devices (Notice No. 14 in 2015). With keen insight for industrial news, Tigermed-Jyton promptly built a Clinical Evaluation Center for medical devices in accordance with regulations and policies. The Clinical Evaluation Center pools senior registration, statistics and clinical research experts at home and abroad. The team has more than 40 members. More than half of them have more than 5 years’ experience in the field. Core members have 10-20+ years’ experience in the field and rich experience in registration approval from the clinical evaluation track. After the Technical Guidelines for Accepting Data of Overseas Clinical Trials on Medical Devices (No.13 Notice in 2018) was issued on January 11, 2018, experts of the center actively study the regulation and prepare the clinical evaluation report with overseas clinical data for the sponsor.
In virtue of rich project experience and professional knowledge, the Clinical Evaluation Center can rapidly and accurately recommend similar medical device(s) to the sponsor, realize technical comparison and difference analysis, collect data information that supports the difference, perform scientific and rigorous data analysis, and prepare high-quality Chinese and English clinical evaluation reports, including
l Exemption report;
l Clinical evaluation report on comparison with similar medical devices;
l Clinical evaluation report for overseas clinical trials.
In addition, based on different strategic needs of different enterprises, the team can provide the most valuable decision reference for the senior leaders of the enterprise through the feasibility evaluation report prepared for the clinical evaluation registration track, and assist them in selecting the most favorable registration track.
To date, the team has served more than 100 medical device companies in the world. Most of global Top 30 medical device companies are clients of the clinical evaluation business of the team. The clinical evaluation business of the team has good word of mouth and reputation and high customer satisfaction.
2. Service Advantages
l Technology Comparison: Having a huge comparison database and rich product experience, the team can accurately determine the similar medical device and key points of technology comparison, and conduct a reasonable evaluation of product performance. High-quality technology comparison output is guaranteed by plenty of experience in material, data and software document preparation. Registration experts have hundreds of registrations of the clinical evaluation track, and different strategies for medical devices in different registration fields.
l Literature Search and Screen: Provide professional and scientific literature search protocol, and quickly search authoritative databases of different countries required by CFDA. The literature screening data record is traceable. Two rounds and above cross-checks ensure the accuracy of the screening results. We also try to contact the author in English to obtain more detailed raw information and try not to miss any valuable evidence.
l Data Extraction and AE Information Summary: The Data Extraction Team has more than 20 members who are proficient in at least two languages and able extract key information from the literature quickly and accurately. Meanwhile, the cross-check of two rounds and above is set. The AE Team can quickly search the AE databases opened by regulatory authorities of each country, collect AEs, SAEs, complaints and risk correction actions related to the product, collate them as required by CFDA regulations, summarize the above information and report to the medical device enterprise.
l Statistical Analysis: Senior statisticians have average experience in evidence-based medicine analysis for more than 5 years, and have statistics-related master degree and above. They are proficient in such statistical software as SAS, skilled at meta-analysis and other qualitative or quantitative statistical analysis methods, and able to scientifically assess the level of evidence of clinical literature and to perform bias analysis.
l Medical Writing: We have writing specialists and medical experts who have 5-10+ years of industry experience and obtained master degree and above from famous medical colleges at home and abroad. They have professional clinical background, rich medical writing experience and successful experience of registration approval. The well-established SOP is strictly followed for writing. Rigorous internal review mechanism is set up to ensure the delivery of high-quality clinical evaluation reports.
3. Development and Achievements
Since its establishment, the Clinical Evaluation Center has established a cooperative relationship with more than 100 famous medical device enterprises at home and abroad. 80% clients are overseas clients. Within two short years, we have quickly and professionally completed more than 180 registration projects related to passive or active medical devices through the clinical evaluation track. More than 20 application fields are covered, including cardiovascular, orthopedics, endocrinology, surgery, stomatology, in vitro reproduction, imaging and neurology.
The team of the center has rich experience in project cooperation, has keen insight on the development trend of the industry, and can accurately interpret current regulations and policies, reasonably mitigate risks, rapidly and efficiently bridge the enterprise and the regulatory authority and fully devote to providing the best and professional clinical evaluation services for medical device companies at home and abroad.
4. Basic Regulations
Document Name | Document No. | Release time | Implementation time |
Regulations on the Supervision and Administration of Medical Devices | No.680 Decree of the State Council | 2017-5-4 | 2017-5-4 |
Administrative Measures for Medical Device Registration | No.4 Order of CFDA | 2014-7-30 | 2014-10-1 |
Technical Guidelines for Clinical Evaluation of Medical Devices | No.14 Order of CFDA | 2015-5-19 | 2015-5-19 |
Technical Review Guidelines for Registration of Medical Devices | / | / | / |
Technical Guidelines for Accepting Data of Overseas Clinical Trials on Medical Devices | No.13 CFDA Notice in 2018/ | 2018-1-11/ | 2018-1-11/ |
Catalog of Medical Devices Exempted from Clinical Trials (Amended) Catalog of IVD Reagents Exempted from Clinical Trials (Amended) | No.94 CFDA Notice in 2018/ | 2018-10-1/ | 2018-10-1/ |
5. Service Process