1. Overview
In accordance with Good Manufacturing Practice (GMP) and Good Supply Practice (GSP), the enterprise should establish and improve a Quality Management System which is applicable to the medical devices manufactured and operated and guarantee its effective operation based on relevant requirements and combining with product characteristics. To manufacture safe and effective medical devices conforming to quality standards, the enterprise must establish a systematic, coordinated and effective quality system which is based on hardware, supported by the document system and assured by personnel.
2. Service Contents
Counseling on clean workshop construction and verification
Counseling on common equipment verification
Counseling on special process verification
Counseling on the design and development process
Counseling on preparation and improvement of quality system documents.
Team building and training for the quality system.
Compliance counseling on QS hardware such as workshop and production equipment.
Training guidance on establishment and execution of the quality system.
Preparation, submission and tracking of QS assessment materials until the assessment is passed, and assessment report obtained.
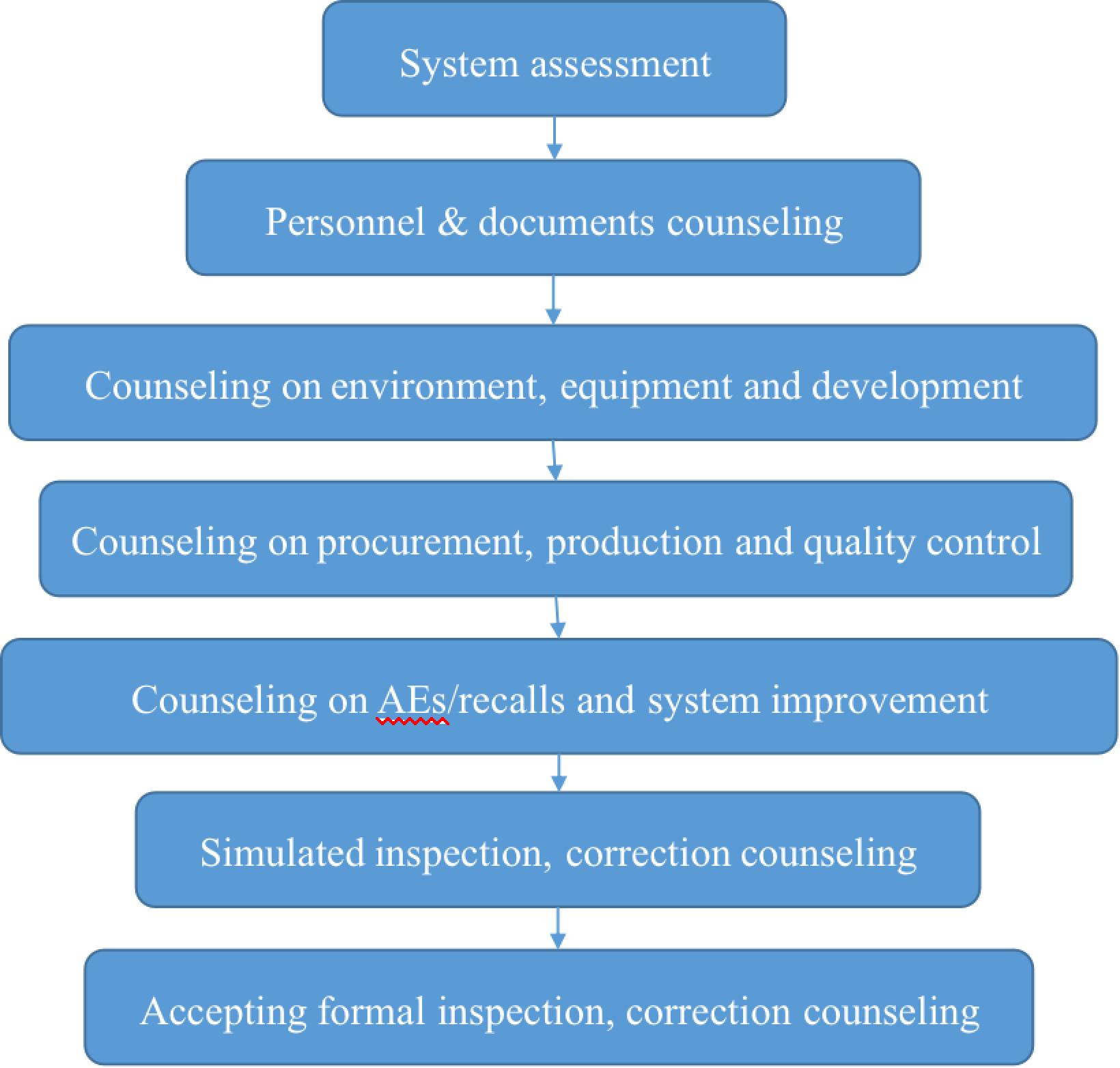
3. Process of System Counseling
4. Team Advantages
Tigermed-Jyton Medical Device Innovation and Development Technology Service Platform has a team which has almost 20 years’ experience in medical device regulations and GMP. Experts of the team participated in the national special supervision and inspection organized by NMPA for several times, and served as the leader of the special supervision and inspection group. They participated in the regulation amendment discussion organized by NMPA for several times, and participated in drafting and amending multiple local supervision and administration regulations for medical devices. They have strong abilities to comprehensively analyze, judge and handle compliance affairs of enterprises which manufacture and operate medical devices. They can provide professional guidance and assistance on establishing and maintaining the quality management system, legally and reasonably mitigating policy risks, and exercising risk control management and performing various verification activities.
5. Reference Regulations and Standards
1. Regulations on the Supervision and Administration of Medical Devices (No.650 Decree of the State Council of the People’s Republic of China)
2. Measures for Supervision and Administration of Medical Device Production (No.7 Order of CFDA)
3. Good Manufacturing Practice (No.64 Announcement in 2014)
4. Good Manufacturing Practice Annex on Sterile Medical Devices (No.101 Announcement in 2015);
5. Good Manufacturing Practice Annex on Implantable Medical Devices (No.102 Announcement in 2015);
6. Good Manufacturing Practice Annex on IVD Reagents (No.103 Announcement in 2015)
7. Good Manufacturing Practice Annex on Customized False Teeth (No.195 Announcement in 2016)
8. Good Manufacture Practice Annex on Stand-Alone Software (No.43 Announcement in 2016)
9. GB/T 19001 Quality management system — Requirements
10. YY/T 0287 Medical devices—Quality management systems—Requirements for regulatory purposes