泰格捷通是泰格医药(股票代码:300347.SZ/3347.HK)全资子公司,成立于2000年,是中国领先的医疗器械(含体外诊断产品)法规事务及临床研究CRO,覆盖医疗器械各领域全生命周期。在过去的20年中我们与来自30多个国家的1900多家医疗器械研发及生产企业建立了长期合作关系,以我们专业的服务能力为客户降低注册风险、缩短项目周期、节约研发经费,推进产品市场化进程。
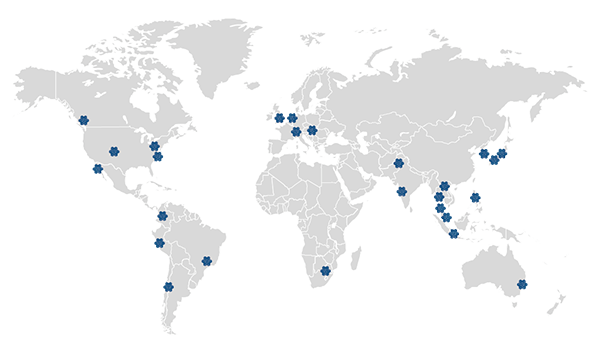
Supported by Tigermed Group, we have more than 180+ offices in major cities on five continents worldwide,covering major medical device markets such as the United States, Europe, and Asia Pacific.

National High-tech Enterprise

ISO9001

CNAS/CMA